Food & Beverage: Lysis-Disruption
APPLICATION PURPOSE
To disrupt yeast cells with the aim of enabling the extraction of valuable compounds of interest for the food and beverage industry like enzymes, pigments, vitamins or bioactive compounds.
CHALLENGES
Breaking down the cell wall is crucial for efficient extraction of intracellular components1. The breaking down can be quite challenging since the yeast cells are protected by a rigid cell wall, which can be resistant to lysis.
HOW DID WE ACHIEVE IT?
Yeast are an attractive and economic source of proteins, enzymes, polysaccharides and even oils. For example, S. cerevisiae is widely used for protein production, including Single Cell Protein (SCP) for animal feed and human diet. It is also used in health supplements and natural flavor compounds for the food industry2. Another yeast, Y. lipolytica, is known for its ability to secrete lipase, which is valuable in the food, pharmaceutical, and environmental industries. It can also produce citric acid under nutrient-limiting conditions and accumulate single cell oil (SCOs), which has potential applications as a cocoa butter replacement3.
Several mechanical methods for cell lysis have been explored such as high pressure homogenization, bead mill and rotor- stator systems. Kinematica is well-known for its rotor-stator equipment which have been proved to disrupt certain cells like the fungi Fusarium, for which isolation of DNA has been performed by means of POLYTRON® PT 1200C 4. However, high pressure homogenization is suitable for a wider range of cell types and can achieve high disruption efficiency.
Kinematica is committed to cutting-edge technologies with the highest efficiency, extending beyond rotor-stator systems. That’s how in collaboration with ETH Zürich, the novel ATOMIX® was born as an alternative for conventional high pressure homogenizers. With its new and patented design for the working chamber, ATOMIX® addresses obstacles that conventional units exhibit.

THE KINEMATICA SOLUTION
The most common working chambers (also called interaction chambers) are divided in two groups: the radial diffuser and the counterjet dispergator5. Those designs of the interaction chambers enable two scenarios: either a fluid jet hits a stationary wall or multiple jets collide with each other. These designs promote flow interactions that can lead to coaleasence, exhibit some dead zones and there are zones in the chamber with higher cavitation.
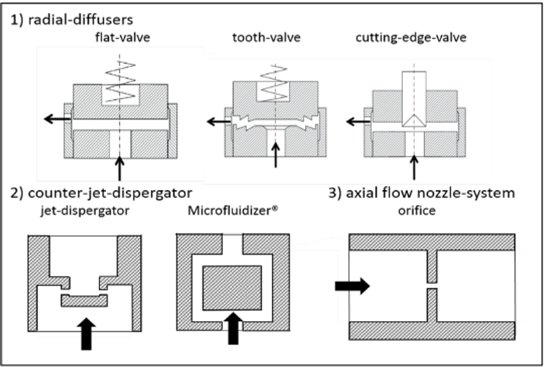
Common interaction chambers in high pressure homogenizers. (Gall et al., 2016)
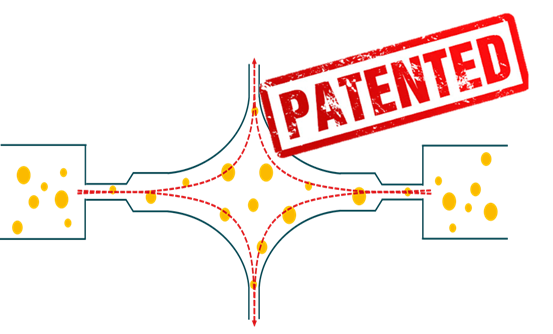
The special and re-engineered design of the working chamber of ATOMIX® promotes a large amount of elongational flow, which is critical to achieve efficient droplet breakup in the case of emulsions at high viscosity ratios, and is beneficial for cell disruption. Also by design the interaction chamber exhibit no dead zones, the cavitation is reduced improving the performance and the flow is controlled leading to improved product quality.
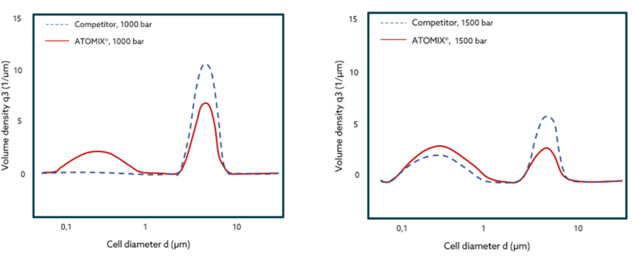
Yeast cells slurry have been processed with ATOMIX® and a popular competitor. The results with ATOMIX® exhibit a higher volume density at smaller cell diameters while operating at the same pressure—after 1 pass. This is translated in more cells being disrupted into smaller particles and therefore, easier extraction processes with higher yields, at shorter time!
Do you have an application with yeast or fungi? Contact us science@kinematica.ch
1 Liu, Dan, et al. “Yeast Cell Disruption Strategies for Recovery of Intracellular Bio-Active Compounds — A Review.” Innovative Food Science & Emerging Technologies, vol. 36, 2016, pp. 181–92, https://doi.org/10.1016/j.ifset.2016.06.017.
2 Ganeva, Valentina, et al. “Extraction of Proteins and Other Intracellular Bioactive Compounds From Baker’s Yeasts by Pulsed Electric Field Treatment.” Frontiers in Bioengineering and Biotechnology, vol. 8, Dec. 2020, p. 552335, https://doi.org/10.3389/fbioe.2020.552335.
3 López-Gómez, José Pablo, and Cristina Pérez-Rivero. “Cellular Systems.” Comprehensive Biotechnology, Elsevier, 2019, pp. 9–21, https://doi.org/10.1016/B978-0-444-64046-8.00067-7.
4 de Nijs, Monique, et al. “Isolation of Fusarium DNA for Molecular Analysis with and without Mechanical Cell Disruption.” Journal of Microbiological Methods, vol. 27, no. 1, Sept. 1996, pp. 13–17, https://doi.org/10.1016/0167-7012(96)00920-7.
5 Gall, Vanessa, et al. “Extending Applications of High-Pressure Homogenization by Using Simultaneous Emulsification and Mixing (SEM)—An Overview.” Processes, vol. 4, no. 4, Nov. 2016, p. 46, https://doi.org/10.3390/pr4040046.