Pharmaceutical: Wet-milling
APPLICATION PURPOSE
Deagglomeration of common APIs used in the pharmaceutical industry like Triamcinolone Acetonide (TAS) and Betamethasone Acetate (BSS) using rotor-stator homogenizers.
CHALLENGES
The particle size of suspended APIs such as Triamcinolone Acetonide (TAS) and Betamethasone Acetate (BSS) can vary depending on the formulation having a wide initial distribution. This makes the particle size reduction process very challenging. Suspension stability, process temperature control and the continuous control of the particle size are crucial parameters for these kind of formulations.
HOW DID WE ACHIEVE IT?
In the pharmaceutical industry, wet milling of APIs is a common practice due to the advantages it brings to the formulations. Wet-milling increases the surface area of the solids, increasing the dissolution rates and the bioavailability. Quite desired parameters when formulating, for example, drugs for injectable administration. Wet-milling is effective in addressing poor solubility in a new drug compound, it is inexpensive compared to other methods and ensures a tight size distribution of the particle size in a very effective manner. On the other hand, the use of wet-milling in the pharmaceutical industry has been proven, among other things, to control nucleation for certain APIs possessing wide metastable zone widths (MSZWs) and thus exhibiting slow nucleation kinetics. Its use has also been shown to reduce the process startup and time to reach the steady state 1.
Certain APIs like Triamcinolone Acetonide and Betamethasone Acetate are quite challenging to reduce the particle size especially when the initial agglomerates are large and when the primary particles show a wide size distribution because of compact density. In these cases a conventional rotor-stator system might not be enough to deagglomerate the particles and reduce their size effectively. Higher shear rates are beneficial to ensure compliance in the applications including these typs of APIs.

THE KINEMATICA SOLUTION
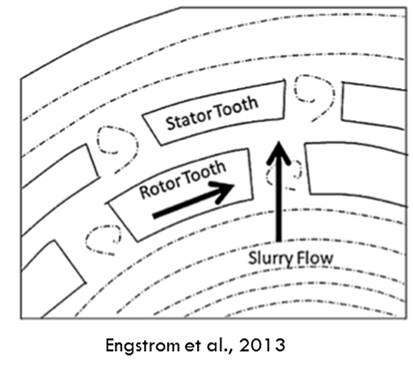
MEGATRON® MT-SHS is an excellent solution for reducing challenging API sizes. Like conventional rotor-stator systems, it operates by creating a channel when the rotor aligns with the stator. As the slurry flows through, the rotor's teeth impact the particles, delivering the energy needed for size reduction.2 When dealing with suspensions, particle size reduction is a combination of shear and impact effects.

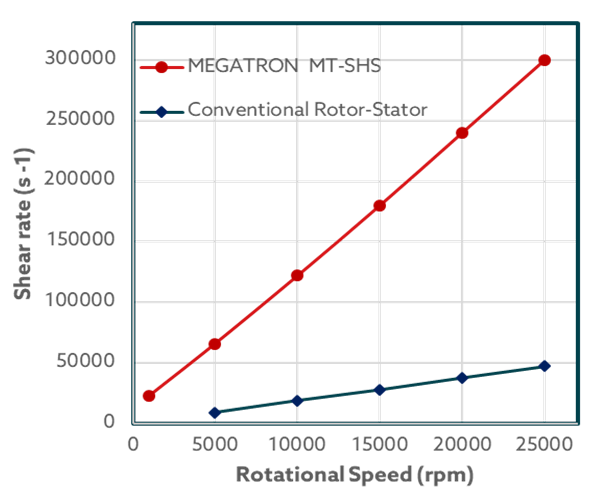
What sets MEGATRON® MT-SHS apart from conventional rotor-stator systems?
It's all about optimizing the shear gap. In typical units, the gap between the rotor and stator is 0.4 – 0.5 mm, but in MEGATRON® MT-SHS, it's narrowed to just 0.2 mm. This reduction directly boosts shear rates, especially since this unit can reach a maximum of 25,000 rpm. In fact, it can achieve shear rates up to 6 times higher than conventional units. This enhanced efficiency guarantees the desired particle size.
In the development and production of pharmaceuticals, the focus is on the efficacy, safety and quality of the product. Production facilities set the strictest regulations and quality requirements, and the required quality characteristics are constantly checked by in-process controls3. The outstanding partnership between KINEMATICA and SOPAT allows not only the particle size reduction but also the inline measurement for fast quality control. This is a great step in process optimization!
By measuring continuously the particle size, the quality of the product is ensured during the whole process, reducing in product lost, reduces processing time and unnecessary production steps for the respective quality off-line measurements. These aspects are crucial considering the impact of the stops in productivity.
Triamcinolone Acetonide and Betamethasone Acetate were processed by means of MEGATRON® MT-SHS and the particle size during the process was evaluated with SOPAT Ma probe along with the U-light attachment. The U-light serves as an alternate mode of illumination for the particles which is especially useful in dense emulsions, suspensions and slurries making it the perfect attachment for measurement of the particle size of Triamcinolone Acetonide and Betamethasone Acetate. The SOPAT probe was used to measure suspension samples before and after homogenization. The gap of the U-light was set approximately to 1mm and continuous stirring at low rpm (ca. 150-200 rpm) was ensured with a magnetic stirrer. Probe control settings like focus position and strobe intensity were changed and set until clear live images with distinct particle boundaries were observed. Live image analysis was started using a standard library workflow for suspensions.
Being an image-based inline particle analysis system, SOPAT measures Feret diameter of the particle. The maximum Feret diameter was taken into consideration for this analysis. The particle size reduction for the Triamcinolone Acetonide and Betamethasone suspensions is shown as follows as number based percentiles.
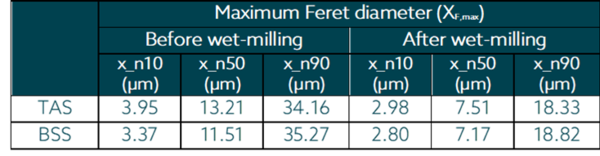

For Triamcinolone Acetonide a particle size of Df,max* of 18.33 µm (starting particle size 34.16 µm) was achieved according to SOPAT results. Meanwhile, for Betamethasone Acetate a particle size of Df,max * of 18.8 µm (starting particle size 35.27 µm). It is important to mention the results from inline photo-optical measurements using SOPAT can be very well correlated with laser diffraction method(widely used in the pharmaceutical industry). However, the results might not have the same numerical value. Correlation can be performed based on the initial particle size.
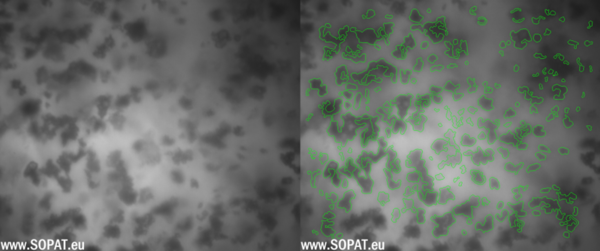
Initial images TAS
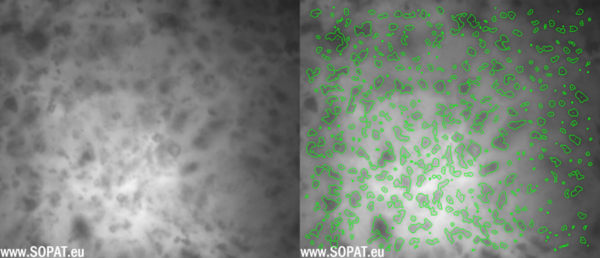
TAS after wet-milling

* 90% number based value
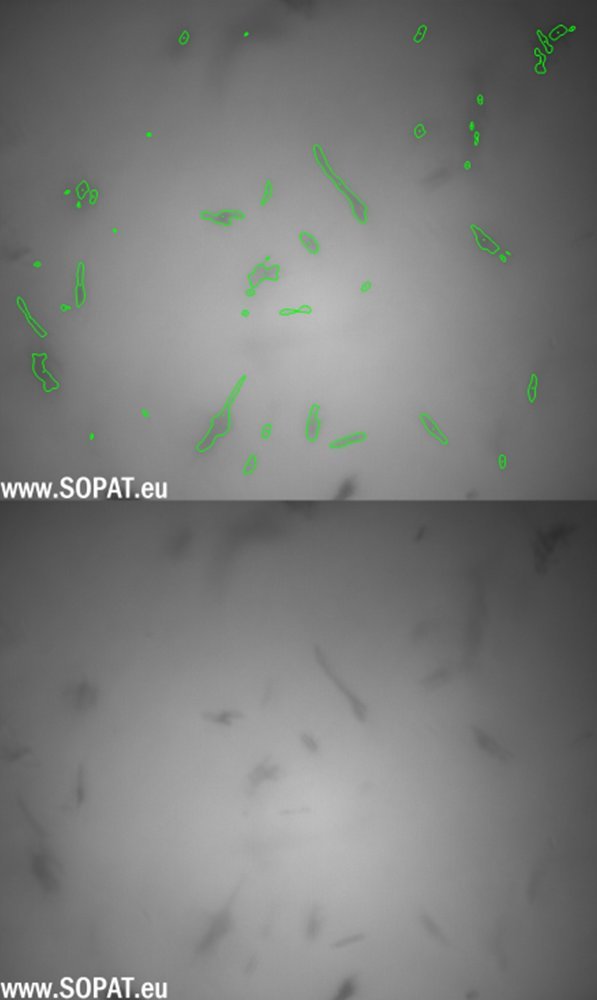
Initial images BSS
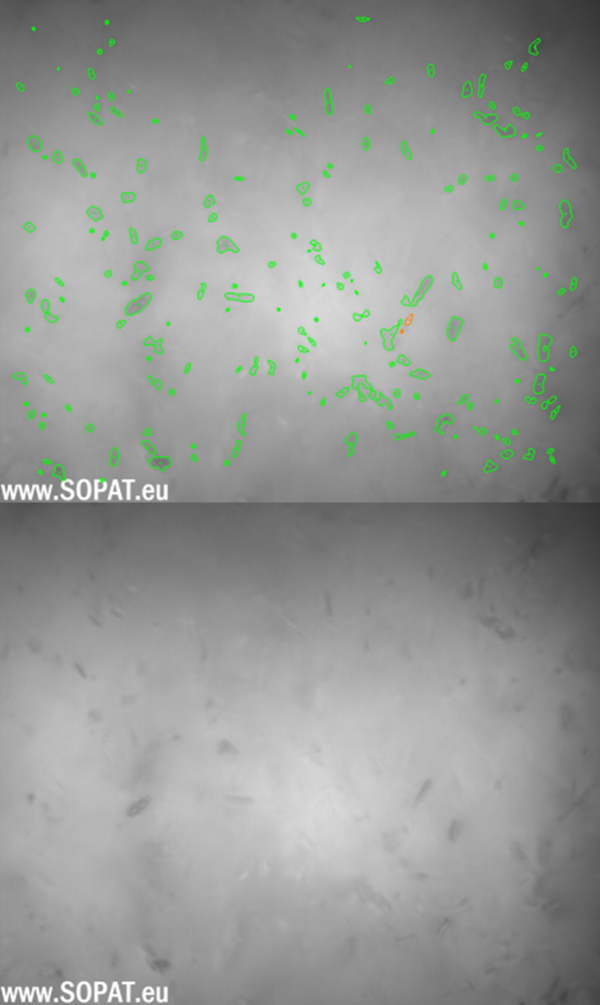

BSS after wet-milling
Unlock the power of SOPAT technology: Dive deeper into Innovation and visit them at https://www.sopat.de/de/
Do you have an application for pharmaceutical APIs? Contact us science@kinematica.ch

1 Yang, Y., Ahmed, B., Mitchell, C., Quon, J. L., Siddique, H., Houson, I., Florence, A. J., & Papageorgiou, C. D. (2021). Investigation of Wet Milling and Indirect Ultrasound as Means for Controlling Nucleation in the Continuous Crystallization of an Active Pharmaceutical Ingredient. Organic Process Research & Development, 25(9), 2119–2132. https://doi.org/10.1021/acs.oprd.1c00209
2 Engstrom, J., Wang, C., Lai, C., & Sweeney, J. (2013). Introduction of a new scaling approach for particle size reduction in toothed rotor-stator wet mills. International Journal of Pharmaceutics, 456(2), 261–268.
3 SOPAT. (n.d.). INDUSTRIES & APPLICATIONS: Pharma & Biochemistry. INDUSTRIES & APPLICATIONS: Pharma & Biochemistry. Retrieved September 14, 2023, from https://www.sopat.de/en/industries-applications/pharma-biochemistry/